An AI-assisted, digital assessment solution designed to reduce the time required to produce accurate kidney biopsy reports for organ transplant viability.
Fusion Organ Donation Suite
Overview
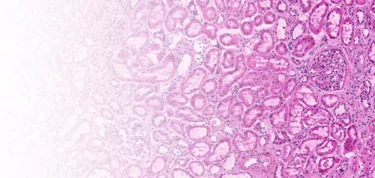
The Techcyte Fusion kidney algorithm helps pathologists digitally assess the viability of kidneys for transplantation.
Using AI and a specialized digital workflow, we help pathologists find and count key structures in the kidney. Kidney biopsy samples are prepared as a frozen section, digitally scanned in using the Grundium Ocus scanner, analyzed by AI and then presented to the pathologist for confirmation. The final report, images of the kidney and the associated internal structures and sent to the surgeon to aid in the final assessment.

Challenge & Solution
Kidney biopsy pathology reviews are ultra time sensitive and if delayed, increase cold ischemic time and the risk of organ discard. Specialized transplantation renal pathologists may not always be available and manually identifying structures such as glomeruli can be time-consuming. The lack of standardization between pathologists can add variability and decrease trust in the assessment of the viability of the kidney.
Techcyte’s AI algorithm automates glomeruli, artery and arterioles identification, with the aim of reducing review time and minimizing subjectivity. It provides visual results, enabling surgeons to quickly and reliably confirm the pathologist’s assessment.
How It Works
4-step process:
Create slides
Biopsy samples are made into slides using the frozen section H&E process.
Scan slides
Slides are scanned using the Grundium Ocus 40 scanner. They are automatically uploaded to the Techcyte platform for AI analysis.
AI Analysis
The AI analyzes the images to locate and enumerate the areas of interest.
Record Results
Results are displayed in a web viewer where pathologists confirm and prepare a report. Finalized findings are shared with surgeons, who make the final decision on whether or not to accept the kidney.
Pre-classified areas of interest
- Normal glomeruli
- Sclerosed glomeruli
- Arteries
- Arterioles
Supported Scanner

Grundium Ocus 40
Features
-

Fusion: The unified digital pathology platform
Built on the state-of-the-art Fusion anatomic and clincial pathology AI platform, unique of its kind -

AI-powered
AI-proposed images of normal and sclerosed glomeruli, arteries, and arterioles, grouped by clas, sorted by confidence -

Glomeruli ratio
The system automatically calculates the ratio of sclerosed glomeruli, allowing surgeons to quickly determine the viability of the kidney for a transplant -

Customizable
Customizable results panel, making it easy to mark additional findings using built-in annotation tools -

Slide-sharing
Easy slide-sharing with multiple recipients -

Slide archiving
Digital archiving & storage of slides
Benefits
Partners

Related Resources
- Press Release

To Boost Organ Transplant Availability, CompuMed to Integrate the Techcyte Digital Pathology Platform
Oct 2023 - Press Release

New Study by The University of Utah and Techcyte Demonstrates the Value of AI in Organ Transplantation
Feb 2023 - Press Release

Techcyte donates to the University of Utah’s Transplant Buddy Fund and previews AI-powered digital organ donation solution
May 2022



