Over the past two decades, parasitology has seen remarkable advances in diagnostic technology. Molecular tools such as PCR, multiplex panels, and sequencing have become widespread, offering sensitivity and specificity for detecting organisms like Toxoplasma gondii, Trichomonas vaginalis, and Plasmodium species. Yet despite their promise, these methods have notable blind spots. Increasingly, experts are warning that an over-reliance on molecular assays risks leaving critical gaps in patient care and accelerating the decline of morphological expertise in the laboratory.
A 2022 commentary in the Journal of Clinical Microbiology titled “Where Have All the Diagnostic Morphological Parasitologists Gone?” by Richard Bradbury (James Cook University) and colleagues, including Dr. Bobbi Pritt (Mayo Clinic), Blaine Mathison (ARUP Laboratories), and Dr. Marc Couturier (NorDx), took a hard look at this issue. Their conclusion was clear: molecular diagnostics, while powerful, are far from complete solutions.
The Limitations of Molecular Testing
Molecular tools face a number of serious constraints:
- Insufficient coverage: Humans harbor at least 848 species of parasites, yet nucleic acid amplification tests (NAATs) exist for only a fraction of the most common species like Giardia, Cryptosporidium, and Cyclospora. This leaves large diagnostic blind spots
- Specimen incompatibility: Common fixatives such as formalin degrade DNA, making PCR unreliable. Fecal samples, with their bile salts and polysaccharides, also interfere with amplification.
- Limited databases: Even when sequencing works, incomplete reference libraries make it impossible to accurately classify certain parasites, leading to misidentifications and confusion in the literature.
- Cost and complexity: Sequencing and multiplex PCR are expensive, require specialized infrastructure, and are often impractical in resource-limited settings.
- Diagnostic uncertainty: Persistence of DNA or antigen after treatment can yield false positives, while mutations in target genes can lead to false negatives. Even widely used malaria rapid diagnostic tests (RDTs) have shown unreliable performance in certain settings.
The commentary warns that over-reliance on molecular tools—at the expense of morphology—has already led to published misidentifications (e.g., Loa loa reported in non-endemic India, Paragonimus westermani in West Africa) and even the erroneous description of new “species” that were later shown to be artifacts.
Why AI provides a better path
Artificial intelligence addresses many of the shortcomings Bradbury and colleagues highlight. Instead of relying on a limited menu of assays, AI leverages digital morphology to recognize all parasites visible on a slide. Unlike PCR, it is not constrained by degraded DNA, incomplete reference databases, or expensive consumables.
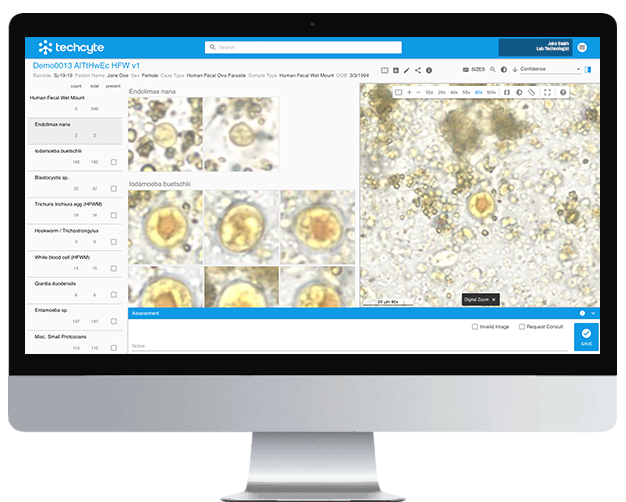
Its strengths include:
- Comprehensive scope: Any morphologically intact parasite can be identified.
- Reliability: AI delivers consistent results, not subject to human fatigue or batch variability.
- Accessibility: Once deployed, AI reduces dependence on expensive consumables, making it scalable across both high- and low-resource settings.
Most importantly, AI preserves the morphological expertise that is at risk of disappearing. Instead of losing the ability to “see” parasites, AI captures, analyzes, and archives morphology, helping train the next generation of laboratorians while producing actionable results today.
How we’re meeting this challenge
At Techcyte, we’ve built our research use only (RUO) platform to directly address the blind spots in current practices and safeguard morphological expertise. What sets us apart is the scale, rigor, and integration of our approach:
- Expert-trained AI: Algorithms trained on hundreds of thousands of expertly annotated parasite images collected from reference labs worldwide give unmatched breadth and accuracy.
- Trusted by leaders: Institutions like ARUP Laboratories, Mayo Clinic, and Quest Diagnostics already use Techcyte’s O&P-assisted RUO screening tools as a lab-developed test, meaning they have validated our platform in some of the most demanding laboratory environments.
- Training and education: Built-in exemplar libraries of every organism type, help labstrain newcomers, transition to digital, and maintain continuing education or certification.
- Unified platform: Beyond parasitology, Techcyte provides a single AI-powered solution spanning bacteriology, cytology, hematology, and anatomic pathology, enabling labs to scale digital pathology across disciplines.
- Regulatory-grade compliance: Granted country-specific regulatory markings*, ISO 13485 certification, and compliance with SOC 2, HIPAA, and GDPR ensure that Techcyte is built to meet the highest global standards.
- Seamless integration: Partnerships with whole slide scanner manufacturers and LIMS/LIS vendors make AIa fully connected part of the lab ecosystem, not a bolt-on.

*Trichrome solution: CE-marked in Europe, cleared for use in Switzerland, and for research use only in the U.S.
*MAF solution: CE-marked in Europe, and for research use only in the U.S.
*Wet mount iodine solution: UKCA and for research use only in the U.S.
Looking Ahead
Molecular diagnostics answer some questions well, but they cannot cover the full spectrum of parasites, patient settings, and clinical needs. AI-powered digital morphology does more.
By combining breadth, speed, and scalability with expert-driven training tools, Techcyte ensures laboratories not only meet today’s diagnostic demands but also preserve the expertise essential for tomorrow.
For labs seeking a path beyond the limitations of molecular testing, Techcyte offers a proven, unified platform that delivers results while cultivating the next generation of parasitology expertise.





